Archived article
Please note that tax, investment, pension and ISA rules can change and the information and any views contained in this article may now be inaccurate.
Why tobacco earnings won’t go up in smoke
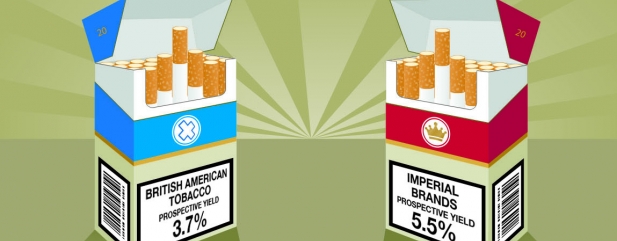
Shares in London’s two listed cigarette titans British American Tobacco (BATS) and Imperial Brands (IMB) sold off heavily following the US Food and Drug Administration’s (FDA) announcement (28 Jul) that it intends to lower nicotine levels in cigarettes to non-addictive levels.
At first glance, the news appears negative for these major contributors to the yield generated by the FTSE 100, although the correction could present a buying opportunity.
The FDA wants to start a public dialogue on how to reduce nicotine levels in cigarettes to non-addictive levels in the US market. Yet no timeline was given and there’s no definition of “non-addictive”, so what this means for the US cigarette market and when is unclear.
Berenberg notes that ‘the FDA by statute is not legally permitted to force the industry to reduce nicotine to zero, and this would be technically very difficult anyway as nicotine is a natural constituent of tobacco’. Furthermore, ‘the imposition of maximum tar and nicotine levels has not had a dramatic impact on other cigarette markets.’
Bulls also argue the FDA’s changes are likely to speed up smokers’ migration to substitute products, an area where large tobacco firms dominate. British American Tobacco’s £41.7bn acquisition of the remaining 57.8% of Reynolds has deepened its presence in the US, though BATS is the largest vapour company in the world with brands including Vype, Ten Motives and Vuse.
Berenberg writes: ‘The announcement is not all “anti-tobacco”. Indeed the harm reduction policy will be very controversial for many in the anti-tobacco lobby, which prefers a “quit or die” approach. The FDA appears ready to distinguish between different “harm” levels and to favour some products over others.
‘This would be a markedly different approach to the “ban/tax them all” approach prevalent in some countries. The policy change will take years to implement, but if the FDA can back this with scientific proof of success it may serve as a model for other countries.’
Important information:
These articles are provided by Shares magazine which is published by AJ Bell Media, a part of AJ Bell. Shares is not written by AJ Bell.
Shares is provided for your general information and use and is not a personal recommendation to invest. It is not intended to be relied upon by you in making or not making any investment decisions. The investments referred to in these articles will not be suitable for all investors. If in doubt please seek appropriate independent financial advice.
Investors acting on the information in these articles do so at their own risk and AJ Bell Media and its staff do not accept liability for losses suffered by investors as a result of their investment decisions.
 magazine
magazine









